

To verify our prediction, the Murphy group bioconjugated a tetrachlorocyclopentadiene ketal it to a peptide and labeled the peptide with trans-cyclooctene dye. The screening revealed tetrachlorocyclopentadiene ketals as highly reactive and stable dienes with promising bioorthogonal potential. Highly accurate computational methods were used to screen potential bioorthogonal cyclopentadiene candidates. Bioorthogonal reactions take place rapidly and selectively in biological environments and enable the study of biomolecules in living systems.

The computational insights into the reactivities of cyclopentadienes inspired us to develop cyclopentadiene as a bioorthogonal reagent. Conversely, donors pre-distort in the opposite direction to maximize the stabilizing effect the hyperconjugative interaction towards an envelope geometry that favors the anti cycloaddition. This envelope geometry resembles the syn transition state geometry and promotes syn selectivity by minimizing the distortion energy required to achieve the syn transition state. Structural analysis of the ground state geometries revealed that ?-acceptors pre-distort the cyclopentadiene into an envelope-like geometry that minimizes the destabilizing effect of the negative hyperconjugation. Experimentally, electron-withdrawing groups provide syn adducts, while electron-donating groups provide anti adducts.
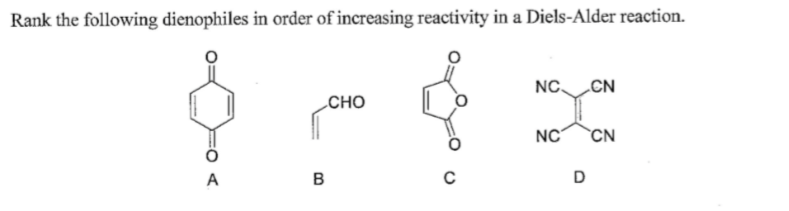
The syn and anti π-facial selectivity of 5-substituted cyclopentadienes is related to the electronic nature of the substituent. In the standard Diels-Alder reaction, there are two components: the diene, which is electron rich, and the dienophile, which is electron poor. The effect is opposite for cyclopentadienes, where hyperconjugative acceptors induce antiaromatic cyclic delocalization of the four π-electrons, destabilizing the diene and promoting reactivity. Hyperconjugative acceptors stabilize cyclopropenes and decrease the reactivity by invoking aromatic cyclic delocalization of the two π-electrons and the reactivity decreases. Substituents at the 3-position of cyclopropenes and the 5-position of cyclopentadienes significantly influence the Diels-Alder reactivity through hyperconjugative interactions of the substituent with the cyclic π-system. The regioselectivity of the Diels-Alder reaction of unsymmetrical dienes with unsymmetrical dienophiles can be predicted by the ortho-para rule. The rapid reactivities of cyclopentadienes result from the minimal distortion required of cyclopentadiene to achieve the envelope-like geometry adopted in the transition state, while the rapid reactivities of cyclopropenes result from reactant distortion and the highly stabilizing orbital interactions present at the transition state. Cyclopentadiene and cyclopropene are unusual, in that they exhibit rapid Diels-Alder reactivity despite their lack of activating electron withdrawing or donating groups. Lastly, cyclic dienophiles like maleic anhydride undergo Diels–Alder reactions to form bicyclic products.Since the discovery of the Diels-Alder reaction in 1928, chemical theorists have pursued a deeper understanding of the factors controlling reactivity and stereoselectivity for this reaction. Herein we present the first comparative computational investigations on the characteristics of DA cycloadditions with anionic dienophiles on the basis of the reactions of ECX anions (E P, As X O, S, Se) with 2 H -pyran-2-one. Next, dienophiles with substituents on both carbons yield stereospecific products, implying that the stereochemistry of the substituents is retained during the reaction. The inter- and intramolecular DielsAlder reactions of. A decrease in the HOMO–LUMO energy gap facilitates the transfer of electrons and increases the reaction rate. o-Quinodimethane derivatives are reactive dienes and can be generated in situ by a number of methods.

So, the reaction rate is influenced by the HOMO–LUMO energy gap.Įlectron-withdrawing groups make the dienophile more electrophilic and lower the energy of the LUMO. Recall that a normal Diels–Alder reaction is driven by the flow of electrons from the HOMO of the diene to the LUMO of the dienophile. One way to speed up the process is by introducing electron-withdrawing groups to the dienophile. H H R H H H R R H H H H H H H H H ( 1 ) ( 2 ) ( 5.8 ) H H H H H R H H H H H cis Fused cyclic dienes are usually the most reactive, particularly if the ring. However, this reaction is slow and requires high temperatures. Let's examine a few typical characteristics of dienophiles.Įthylene is the simplest dienophile that reacts with 1,3-butadiene to form cyclohexene. Alkenes, substituted alkenes, and alkynes are some of the commonly used dienophiles in Diels–Alder reactions. The Diels-Alder reaction is both a 1,4 addition or ethene to 1,3-butadiene and a 1,2 addition of butadiene to ethene.


 0 kommentar(er)
0 kommentar(er)
